Intensive Research Sprint for Pharma
Goals
• To identify core areas of concern around usability
• To understand key user behaviours, thought processes and use cases
Methods
Interactive prototype, concept and usability testing, wireframes, System Usability Scale, interviews and NPS surveys
Challenges
Logistical challenges around conducting in-depth research within a short sprint
Project Overview
In-depth testing with 21 existing users including key industry players from Pfizer, Mckinsey and Roche
Interactive prototypes across various journeys to allow contextual testing experience
Comparing existing and proposed designs in each session to drive rich discussions
Introduction
At Evaluate, we embarked on a major re-platforming initiative triggered by the knowledge that only 2% of our extensive pharmaceutical data was being accessed by users. This challenged our position as a market leader and cast uncertainty regarding the sustainability of our core offering.
As the sole designer, working alongside the VP of product, data analysts and devs, I was perfectly positioned for research including gleaning insights from long-standing team members and of course, end users.
Overview & Key Method
This was an intensive research project, where I engineered a 4 week user testing sprint comparing the existing version (2.0) of our platform to my prototypes of potential, helping us to identify core behaviours and pain points, and also drive discussion.
This sprint involved 21 participants, over half being power users, and others being frequent long-term users, joining us for an in-call session with guided interactive prototypes. I had gamified the test and based it on a realistic use case, which not only contextualised the flow but also showcased the proposed key shifts in our platform (search, tabular data management, report building, alerts etc.)
Opportunities for feedback were open throughout the call, followed by an interview after the test and a post-session survey by email allowing further expansion. My analysis documented both in-call and survey results, which sometimes varied, demonstrating the importance of reducing influence in research.
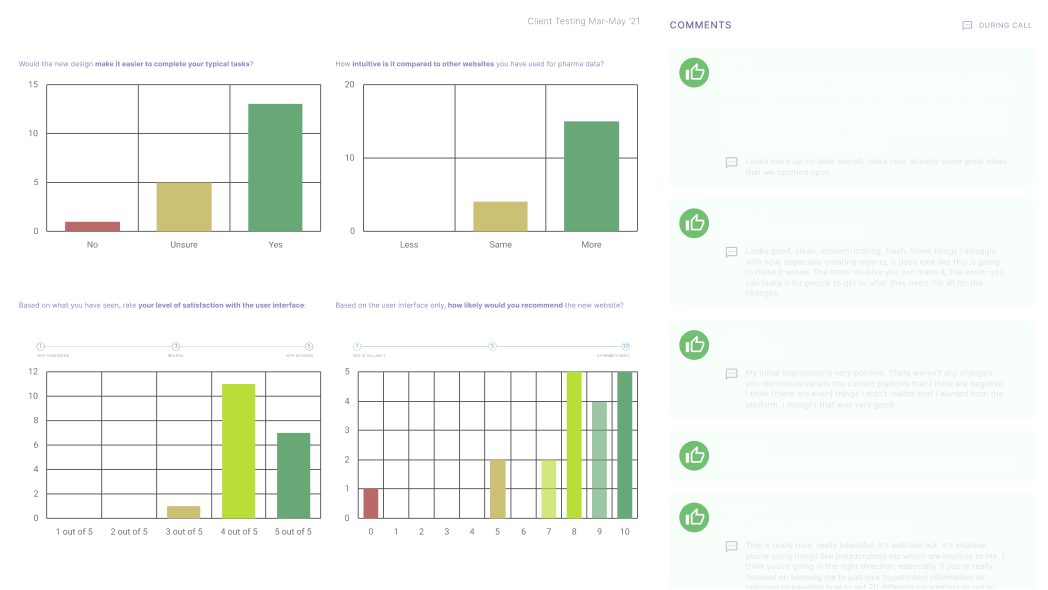
Conclusion
Following several weeks of engagement, I gained a profound understanding of our end users, from power users to ‘general’, their varying challenges and unmet needs. These insights, qualitative and quantitive, enabled me to cluster and identify patterns and eventually devise a strategy. I invite you to browse my full report below:











